Researchers at Washington University School of Medicine in St. Louis have discovered that von Willebrand factor (VWF), which helps the body round up platelets to form clots at injury sites, needs an acidic environment to complete its development. Their findings were published in the July 2011 issue of the Journal of Biological Chemistry.
VWF is a clotting protein whose main function is to bind to platelets, collagen and factor VIII (FVIII), another clotting protein, keeping it in the circulation longer. When a blood vessel is injured, bleeding results. VWF helps platelets, small cell fragments, stick together at the site to plug the hole in the blood vessel and form a stable clot, which stops the bleeding.
In people with von Willebrand disease (VWD), the VWF in their blood is either defective or deficient. These new findings may shed some light on how specific mutations alter the structure or function of the VWF protein. “It is likely that understanding this process will help us understand why some patients have the disease and others don’t,” says Evan Sadler, MD, PhD, professor of medicine at Washington University, and co-author of the study.
How VWF Is Stored and Synthesized
VWF is produced in the endothelium, the cells forming the inner lining of blood vessels throughout the body. It is stored in secretory granules called Weibel-Palade bodies, where it is released when the body undergoes stress or is exposed to the synthetic hormone desmopressin acetate (DDAVP).
But because VWF is such a large protein, endothelial cells can’t easily package and transport it. “The cell solves this problem by assembling VWF in two stages,” Sadler says. During the first stage, a precursor component is made in the endoplasmic reticulum, a structure in the cell that synthesizes proteins and lipids. “It then ships it to the Golgi and Weibel-Palade body, where it is packaged, assembled and coiled like a rope.”
How VWF Unfurls
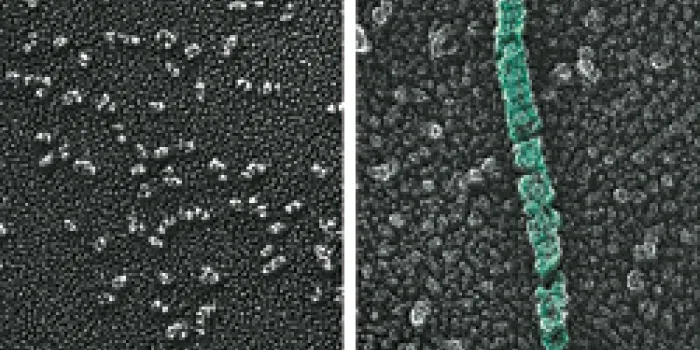
To be effective, VWF has to link into long chains, called multimers, which are stored wound up into tubes. This shape allows VWF to unfurl at the injury site, lassoing platelets that help in clot formation. To form these chains, VWF must have an acidic environment. Sadler and his colleagues hypothesized that histidine, an essential amino acid, senses the changes in pH, which is the measurement of the acidity and alkalinity of a solution. Moving from the neutral environment of the endoplasmic reticulum to the increasingly more acidic environments in the Golgi and Weibel-Palade body causes histidine to gain a positive charge. That charge then prompts the VWF to assemble its long chains.
To pinpoint which histidines were responsible, the researchers compared the DNA sequences of VWF in a variety of animals, from mammals and birds to fish. “If the sequence is preserved in all those animals, then it has a chance of being important either for the structure of the protein or for a process like pH-dependent assembly,” Sadler says. Of the 13 histidines that were in the same location from one species to the next, two were vital to VWF synthesis. When the researchers substituted other amino acids without a positive charge for the histidines, VWF did not form chains.
“There are mutations that affect biosynthesis, storage, multimer assembly and packing into the Weibel-Palade bodies,” says Sadler. Knowing which mutation a patient has might help researchers use gene therapy techniques to develop drugs that restore VWF and the body’s clotting mechanisms. However, it is not known if these findings will directly affect treatment for VWD in the future.